Regenerative medicine has traversed remarkable innovations and advancements, catalyzing a paradigm shift in therapeutic interventions. One such remarkable innovation is the Juventix Plasma Bio-Incubator. Engineered with meticulous precision, the Juventix Bio-Incubator facilitates the synthesis of Platelet-Rich Fibrin (PRF) from an autologous sample of Platelet-Rich Plasma (PRP), marking a significant enhancement in regenerative medicine by producing a stable and effective PRF sample, something that conventional aluminum block “dry bath” heaters an unable to achieve. This article endeavors to unveil the distinguishing features and profound benefits of utilizing the Juventix Plasma Bio-Incubator in comparison to conventional aluminum block “dry bath” heaters.
 Aluminum block heaters, or dry baths, were originally designed to heat samples of tissue, such as cheek swabs, to stimulate a rapid culture. This is a device you may encounter in an urgent care setting to perform a rapid flu test for example. These devices were never designed for utilization in regenerative medicine and are not engineered to maintain the integrity of blood plasma samples for clinical use. The products you may find marketed for sale today are inexpensive devices, primarily sourced from China, that are not manufactured in FDA registered CGMP facilities, and do not carry CE or ISO clearance. An example of one such device is pictured here.
Aluminum block heaters, or dry baths, were originally designed to heat samples of tissue, such as cheek swabs, to stimulate a rapid culture. This is a device you may encounter in an urgent care setting to perform a rapid flu test for example. These devices were never designed for utilization in regenerative medicine and are not engineered to maintain the integrity of blood plasma samples for clinical use. The products you may find marketed for sale today are inexpensive devices, primarily sourced from China, that are not manufactured in FDA registered CGMP facilities, and do not carry CE or ISO clearance. An example of one such device is pictured here.
Unlike these “knock off” devices, The Juventix Plasma Bio-Incubator is manufactured in an FDA registered facility and has been granted CE & ISO clearance. This device boasts a uniquely engineered ceramic heating element regulated by a computerized control system and air-cooling technology to ensure safe and effective gradual heating and cooling. This innovation ensures that the PRP sample is meticulously safeguarded against excessive heat or rapid heating, which could compromise the integrity of the cells by lysing the bi-lipid outer membranes. Unlike the conventional aluminum block heaters, which expose the samples to direct and unregulated heat, the Juventix Bio-Incubator is adept at preserving cellular and protein integrity.
 In fact, the function of the Juventix Plasma Bio-Incubator is so unique that it has been granted a utility patent by the USPTO (U.S. Patent No. 17,861,764). The patented design features syringe bays lined with nylon insulant. This feature ensures that the PRP samples benefit from equal and mild heat exposure, delicately stimulating cellular metabolism. Consequently, thrombosis is gently triggered, enabling the platelets to aggregate and coagulate efficiently. The Juventix method facilitates an enhanced activation of the platelets, leading to the release of unique growth factors. This culminates in the creation of an autologous regenerative solution enriched with anti-inflammatory properties, magnifying its therapeutic efficacy in a myriad of clinical applications.
In fact, the function of the Juventix Plasma Bio-Incubator is so unique that it has been granted a utility patent by the USPTO (U.S. Patent No. 17,861,764). The patented design features syringe bays lined with nylon insulant. This feature ensures that the PRP samples benefit from equal and mild heat exposure, delicately stimulating cellular metabolism. Consequently, thrombosis is gently triggered, enabling the platelets to aggregate and coagulate efficiently. The Juventix method facilitates an enhanced activation of the platelets, leading to the release of unique growth factors. This culminates in the creation of an autologous regenerative solution enriched with anti-inflammatory properties, magnifying its therapeutic efficacy in a myriad of clinical applications.
In contrast to conventional methodologies utilizing aluminum block heaters, the Juventix Plasma Bio-Incubator fosters the production of PRF that stimulates osteoblast activity. This facilitates the proliferation of bony tissue, thus being immensely beneficial in scenarios such as bone fracture repairs, and loss of hyaline cartilage in advanced degenerative joint disease, such as Osteoarthritis of the knee(s). The PRF produced also fosters an anti-inflammatory immune response by orchestrating the release of cytokines like interleukin (IL)-1 receptor antagonist, IL-4, IL-6, IL-10, IL-11, and IL-13.2 This makes it quintessential in mitigating inflammation, reducing swelling, and alleviating pain.
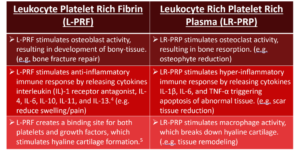
Furthermore, the patented Plasma Bio-Incubation technology pioneered by Juventix Regenerative Medical revolutionary method ensures the PRF serves as a potent binding site for both platelets and growth factors, stimulating cartilage formation and thereby elevating its therapeutic potential across a variety of orthopedic applications, as well as utilization as a natural alternative to dermal fillers in aesthetics.
Diverging from the unregulated and detrimental heating sources synonymous with aluminum block heaters or dry baths, the Juventix Plasma Bio-Incubator stands as a beacon of innovation and efficiency. It mitigates the risks of cell lysing and protein denaturation, thereby preserving the integrity of cytokines and growth factors intrinsic to PRP samples. This functionality has been proven in third-party laboratory testing that confirms the Juventix Plasma Bio-Incubator does not deteriorate, detriment, or destroy these essential cellular components that lend PRF it’s therapeutic potential in clinical application (lab study available by request).
To explore the transformative potentials of the Juventix Plasma Bio-Incubator, and to revolutionize your therapeutic outcomes contact Juventix Regenerative Medical for more information, to schedule a product demonstration, or place an order today: Call: (866) 693-4777 or Email: hello@juventix.com or Visit: www.juventix.com today!
Don’t be fooled by cheap “knock-off” products! Elevate your clinical practice to the zenith of innovation and therapeutic excellence with the Juventix Plasma Bio-Incubator.
Sources:
1) Grecu AF, Reclaru L, Ardelean LC, Nica O, Ciucă EM, Ciurea ME. Platelet-Rich Fibrin and its Emerging Therapeutic Benefits for Musculoskeletal Injury Treatment. Medicina (Kaunas). 2019 May 15;55(5):141. doi: 10.3390/medicina55050141. PMID: 31096718; PMCID: PMC6572609. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6572609/
2) Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007 Spring;45(2):27-37. doi: 10.1097/AIA.0b013e318034194e. PMID: 17426506; PMCID: PMC2785020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2785020/
3) Bai MY, Wang CW, Wang JY, Lin MF, Chan WP. Three-dimensional structure and cytokine distribution of platelet-rich fibrin. Clinics (Sao Paulo). 2017 Feb 1;72(2):116-124. doi: 10.6061/clinics/2017(02)09. PMID: 28273236; PMCID: PMC5304401. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5304401/
4) Schär MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015 May;473(5):1635-43. doi: 10.1007/s11999-015-4192-2. PMID: 25690170; PMCID: PMC4385378. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4385378/







