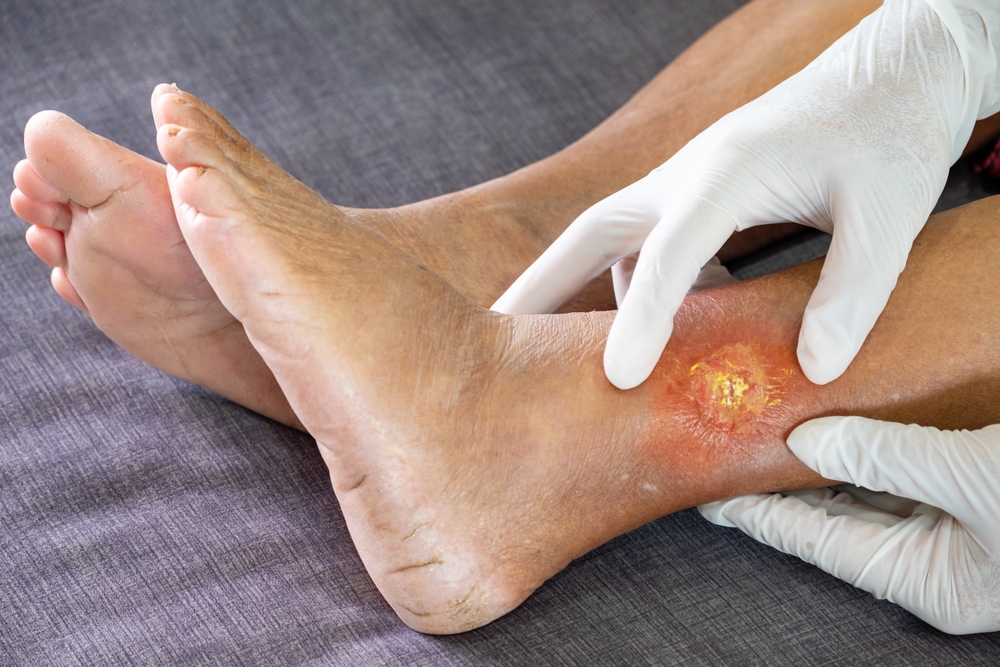
Chronic wounds are defined as wounds that do not heal and remain open after four weeks. In 2014, more than 8 million Medicare beneficiaries suffered from chronic wounds. The associated cost of treatment was estimated to be as high as $50 billion annually. The wound care market is growing at a rate of 4.6% per year and estimates for 2023 to 2031 are 5.4%.
Primary causes of chronic wounds are diabetes, peripheral vascular disease, immobilization or immobility and arterial venous diseases. Most chronic diseases can have secondary chronic healing problems leading to non-healing chronic wounds.
Chronic wounds, no matter what their etiology, share a common pathway of compromised circulation which causes skin breakdown, bacterial colonization and impaired tissue repair processes. The pathology of chronic wounds is complicated and depends on the disease state, but all chronic wounds have a commonality of reduced blood flow, decreased energy support resulting in ischemia and hypoxia. The reactive oxygen species and inflammation are activated by ischemia and hypoxic tissue and this combination retards the healing process.
Besides the damage caused by inflammation, wound infection further disrupts the healing. Normal healing inflammation is a normal part of the overall process to eliminate pathogens, senescent cells and promote proliferation and remodeling. However, in chronic wounds, the long term inflammation promotes more ischemia and necrosis.
Traditional treatment consists of regular wound cleaning and dressings. Classically, saline dressings and Vaseline gauze are the most common dressings. These have been proven to be cost effective because of their safety, efficacy and universality.
Hydrogel dressings comprise a widely used and growing market in clinical treatment. They can provide a moist environment for gas exchange, protect against bacterial pathogen invasion and promote angiogenesis. Most studies have found hydrogels to have satisfactory effectiveness but the limiting factor in their use is the significant cost.
Metallic based dressings are also used in chronic wound healing. Silver based dressings have antibacterial properties, modulation of inflammation and wound healing are all qualities of these dressings. However, the long-term use has safety issues concerning antigenicity in the human and environmental issues. Cost is also a factor in the use of these products.
There is also some biomaterial dressings used in wound management but there is lacking clinical evidence in the efficacy and safety of these class of dressings. Long term clinical experiments are lacking.
Platelet rich plasma is an autologous blood concentrate that modulates tissue repair and regeneration. PRP is rich in cytokines, growth factors and bioactive proteins that has been proven clinically to repair damaged bone, cartilage and other tissues. PRP releases biologically active factors from its alpha granules into the microenvironment. These substances initiate the
hemostatic cascade stimulating the revascularization and tissue regeneration. Neovascularization is critical to reconstruct the blood supply and support the activity of tissue regeneration.
The regenerative properties are well known. They are due to the multiple growth factors released. These include platelet derived growth factor, epithelial growth factor, vascular endothelial growth factor, transforming growth factor and insulin like growth factor. Each one of these growth factors contribute therapeutic effects and stimulate repair and regeneration.
PRP contains immunological mediators, enzymes and suppressor agents that regulate the bacterial population in wounds. This aids in the restoration of healing and tissue regeneration. Leukocytes are critical in this process.
Inflammation, coagulation, epithelialization, granulation tissue formation and tissue remodeling are all components of the healing process. Platelet adhesion at the site of the wound, stimulates activation then aggregation. The fibrin clot formed attracts various cell populations to the site. The platelets undergo degranulation liberating multiple bioactive proteins which causes inflammation. This inflammation attracts neutrophils and macrophages. Reactive oxygen species are produced causing peroxidation of the lipid components and alterations in various antioxidant systems. The disparity between the oxidative stress and antioxidative systems is responsible for the occurrence of cellular senescence or lack of ability to respond and repair.
Chronic wound healing can be inhibited by bacterial contamination. This is prevented or treated by the antimicrobial properties and immunological properties of PRP. Leukocytes contained in PRP are recruited to the site and trigger signaling pathways that phagocytose and kill microorganisms. Studies have proven that after activation, platelets release kinocidins such as CXCL$, CXCL7 and CXCL5. These have antibacterial and antifungal properties. (Kinocidins are antimicrobial chemokines associated with platelets) Reactive oxygen species generated by platelets are capable of adhering to, phagocytosing and aiding in the antibody dependent cellular cytotoxicity.
Antimicrobial Effects of Platelet Rich Plasma and Platelet Rich Fibrin: A Scoping Review
Cureus 2023 Dec;15(12): e51360 PMID 38292974
This study examined and reviewed peer reviewed articles both in vitro and in vivo from 2000 to 2023. 612 articles were examined. Ultimately, 12 articles were selected for inclusion in this review which focused on the antibacterial effects and wound healing properties of PRP and PRF.
Discussion
Over the past 20 years, a lot of research has been done on the regenerative potential of platelet concentrates. Only a few findings about their antimicrobial effects in the literature are currently available. Bacterial infection is the most serious complication that hinders tissue regeneration and wound healing. Bacteria can penetrate and colonize the wounds underlying tissues even after stringent disinfection. The dynamic interaction between proteolytic enzymes, bacterial exudates that are abundant in toxins, and persistent inflammatory response has the capability
to modify the fundamental cellular mechanisms that are crucial for both the expansion of cells and the process of wound healing. Our objective was to scrutinize the research.
Is PRP Bactericidal or Bacteriostatic?
PRP exhibits both bactericidal and bacteriostatic properties, therefore it can kill bacteria and inhibit growth. The effectiveness of PRP in reaching the minimum inhibitory concentration necessary to halt bacterial replication depends on various factors. These include bacterial load present in the wound, the overall health of the host, the type of bacteria and the total quantity of PRP administered. In instances where PRP dosage is insufficient, initial inhibition of bacterial growth may occur, but eventual bacterial overcoming is likely as the antimicrobial effects of PRP diminish over time. Numerous studies suggest that a continuous administration of PRP throughout the wound healing process yields greater benefits.
Does the Use of Various Agents Provide a Synergistic Effect?
Bielecki et al have identified a subset of platelet antimicrobial proteins that exhibit characteristics of chemokines and possess inherent antibacterial effects. These proteins have the ability to work synergistically with conventional antibiotics while minimizing the risk of bacterial resistance development. Platelets possess angiogenic properties and the emergence of new blood vessels at the site of the wound could facilitate the distribution of antibiotics while promoting the natural flow of blood contributing to the healing process.
What is the Mechanism by Which PRP Exhibits Antibacterial Activity?
The antimicrobial proteins and peptides of innate immune defense found in platelets, along with complement and complement binding proteins present in the alpha granules, have been suggested as the contributors to the antimicrobial action of PRP. It has also been proposed that platelets directly engage with microbes and antibody dependent cell cytotoxicity while WBCs directly kill bacteria produce myeloperoxidase, activate the antioxidant responsive element and mount an antigen specific immune response.
Can PRP and PRF Exhibit Antimicrobial Properties?
Most authors agree that platelet preparations, such as PRP and PRF, show varying levels of effectiveness against common wound bacteria like MRSA, MSSA, E.coli. K pneumonia, E faecalis, P aeruginosa and others. The complexity of the wound environment, often characterized by polymicrobial infections, makes therapy effectiveness dependent on both the wound type and the patients overall health.
Implications
The findings of this review are particularly relevant in the context of infection control. These properties suggest PRP and PRF could be valuable tools in managing wound infections especially in cases where traditional antibiotics are less effective due to resistance. The potential synergistic effect of PRP and PRF with antibiotics presents a promising avenue for enhancing the treatment of wound infections.
The author notes: “It is recommended to utilize an FDA approved autologous platelet separator system, moreover, using appropriate activation agents”.
J Pers Med 2023 Feb 27;13(3):430 PMID 36986311
Objective: Many clinical trials have applied Platelet Rich plasma dressings to treat wounds that have stopped healing, which are called chronic wounds. However, the clinical efficiency of PRP dressings is still controversial. We conducted this study to compare PRP dressings with normal saline dressings in treating chronic wounds.
Conclusion: Pooled results showed that the complete healing of the PRP group was significantly higher than that of the saline group at 8 and 12 weeks. In addition, there were no significant differences in wound infection and adverse events. The PRP dressing could enhance the prognosis of chronic wounds.
Adv Health Mater 2024 Feb 2: e2303930 PMID 3806618
Online ahead of print
This system of chitosan, B-glycerophosphate, cellulose, graphene oxide and platelet rich plasma was used on chronic infected wounds. It was placed under infrared light. It was found to improve blood circulation and skin recovery. Antibacterial properties were noted especially against Pseudomonas aeruginosa.
The conclusion was this could be a promising dressing for the treatment of chronic wounds.
Juventix Regenerative Medical is an industry leader in the regenerative medical field. Our Platelet Rich plasma Kits are designed for safety, sterility and effectiveness. Our kits are scientifically manufactured to provide a platelet concentrate, devoid of red blood cells with a minimum number of leukocytes, critical to the regenerative process.
Juventix Regenerative Medical offers a LED Activator to active the platelets and begin the regenerative process. The activation is a critical step in the release of cytokines, growth factors and bioactive proteins from the alpha granules on the platelets and is accomplished with LED light. This negates the use of chemical additives such as calcium chloride, thrombin of collagen. This mode of activation by LED light provides sustained growth factor release versus older methods of activation while adhering to the minimal manipulation guidelines of the FDA.
Juventix Regenerative Medical supplies a bio-incubator to transform the Platelet Rich Plasma to an Injectable Platelet Rich Fibrin. The Platelet Rich Fibrin, commonly called the “second generation of platelet products” has different cytokines and growth factors than the original PRP. These different cytokines provide and anti-inflammatory environment and can be used confidently in inflammatory conditions.
Juventix Regenerative Medical has been providing goods, services and devices for the regenerative professional for years. Our DermaMed LLLT has multiple spectrums of light including infrared and blue which has anti-infective properties. In combination with our PRP and PRF, the DermaMed is an added resource when treating chronic infected wounds.
Regenerative Regards,
Dr. Robert McGrath