Why Regenerative PRP + Biotin and Needle-Free Topicals Offer a Safer Path Forward for Hair Restoration:
Glycolic Acid on the Scalp: Why Clinicians Should Pause Before Adopting the Trend
Glycolic acid has migrated from facial peels into hair-care marketing, where it is promoted as a “scalp exfoliant” that can supposedly stimulate hair growth. In practice, the data tells a different story…
Consumer-facing brands and blogs frequently highlight theoretical benefits, removal of sebum, desquamation of keratin, and flake control, but acknowledge that there is no robust clinical evidence that glycolic acid reverses androgenetic alopecia (AGA) or other common causes of hair loss.
Dermatology-oriented reviews emphasize that:
- Evidence for hair regrowth is limited to anecdote and speculative mechanisms rather than controlled trials.
- Over-use or high concentrations can cause barrier disruption, erythema, burning, and chemical irritation, particularly on already-inflamed scalps.
- Repeated irritation may weaken hair shafts at the ostia and exacerbate shedding, especially when acids are layered with other active agents or left on too long.
For patients already anxious about hair loss, adding an unpredictable irritant on top of AGA, telogen effluvium, or traction alopecia can be counter-productive. Clinically, glycolic acid may have a niche in managing scale or seborrheic dermatitis, but it is not a hair-restoration therapy.
Beyond Finasteride and Caustic Acids: Why Providers Are Moving Toward Regenerative Options
At the same time, regulatory and pharmacovigilance developments are forcing a re-evaluation of systemic hair-loss drugs. In May 2025, the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) confirmed suicidal ideation as a side effect of finasteride 1 mg and 5 mg tablets, widely used for AGA, and implemented new risk-minimization measures.

While these drugs remain important tools, many primary-care, aesthetic, and regenerative medicine clinics are asking:
“Can we deliver meaningful hair regrowth without chronic systemic exposure or aggressive chemical peels?”
Current evidence suggests the answer is yes! Through autologous and biologic regenerative strategies such as Platelet-Rich Plasma (PRP), Biotin (B7), and advanced topicals that leverage the patient’s own growth factors, as well as potential allogeneic sources (exosome-augmented protocols).
PRP for Hair Loss: From “Experimental” to Evidence-Based Standard of Care
Multiple randomized controlled trials and meta-analyses now support the efficacy of platelet-rich plasma (PRP) in androgenetic alopecia:
- A recent meta-analysis of randomized trials found that PRP significantly increased hair density and hair count versus baseline or placebo, with a favorable safety profile.
- Head-to-head and adjunctive studies suggest PRP can match or outperform topical minoxidil in some cohorts and enhance outcomes when combined with standard pharmacotherapy.
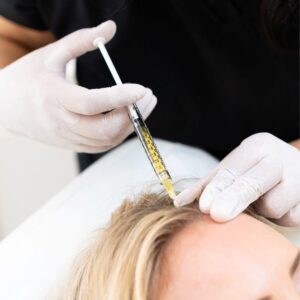
PRP infusion at the follicular level activates key signaling pathways, VEGF, PDGF, IGF-1, to enhance dermal papilla function and transition resting follicles back into the active anagen phase.
Mechanistically PRP delivers supraphysiologic concentrations of platelet-derived growth factors (PDGF, VEGF, IGF-1, TGF-β and others) that:
- Prolong the anagen phase
- Enhance perifollicular microcirculation
- Modulate inflammation and stimulate dermal papilla cell proliferation
This regenerative profile directly addresses key elements of hair-follicle miniaturization, in contrast to glycolic acid, which primarily functions as a keratolytic surface agent.
Biotin (B7) + PRP: A Patented Innovation from Juventix
Biotin plays a recognized role in keratin infrastructure and has been associated with improved hair parameters in deficient patients. However, oral supplementation alone is limited by gastrointestinal degradation and variable adherence.
To address this, Juventix Regenerative Medical developed a one-step PRP harvesting kit that integrates liquid biotin directly within the PRP collection tube. The U.S. patent application “Kit Including Biotin for Harvesting Platelet-Rich Plasma” (US 2023/0038636 A1) describes a PRP tube containing:
- A gel separator to partition red blood cells and granulocytes
- An anticoagulant above the gel layer
- Liquid biotin in injectable form located below the gel separator, enabling simultaneous preparation of PRP and biotin in a closed, chemical-free system for hair-loss treatment.
In October 2025, the United States Patent and Trademark Office issued a Notice of Allowance for this non-provisional utility patent application (Application No. 17/877,268), confirming that the claims are allowed for issuance as a U.S. patent.
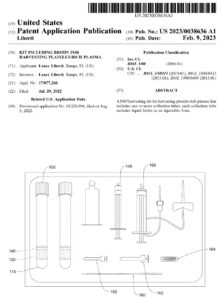
For clinicians, this means:
- A procedural-ready, patent-protected PRP + Biotin kit designed specifically for hair restoration
- Closed-system preparation that minimizes manipulation and contamination risk
- The ability to deliver growth factors and biotin directly to the follicular microenvironment in a single step
In a therapeutic landscape crowded with unregulated topicals and off-label acid treatments, a clearly defined, protected methodology offers both scientific and medico-legal advantages.
From In-Office to At-Home: Needle-Free Delivery with PRiVIVE™ Topical Serum
Clinics also need a way to extend in-office PRP outcomes into the home setting without asking patients to inject themselves or rely on harsh chemicals.
This is where PRiVIVE™ Topical Serum functions as a bridge between regenerative medicine and advanced cosmeceutical delivery:
- PRiVIVE™ uses a proprietary, clinically demonstrated self-contained delivery technology (ALM DT) that enhances dermal permeation and cellular utilization of active compounds without external devices. Its mechanism has been likened to a form of “chemical electroporation” that transiently increases transcellular and intercellular transport without damaging tissue.
- In an ex-vivo human skin model (Mattek), platelets carried within a platelet-rich fibrin matrix plus ALM DT crossed the stratum corneum by 4 hours, reached the basal cell layer by 24 hours, and produced a four-fold increase in fibrin at the basal membrane along with elevated IL-6, indicating biologic activity, without observable histologic damage.
Combined with PRP, this suggests a practical, clinically tested pathway to “inject PRP without needles” by applying a patient-specific PRP-enriched PRiVIVE serum onto intact scalp or skin immediately after in-office procedures.
PRiVIVE is also formulated with additional regenerative ingredients that support scalp and skin health:
- Centella asiatica extract (Gotu Kola) – promotes fibroblast proliferation, collagen synthesis, and tensile strength of healing skin
- Palmitoylethanolamide (PEA) – a lipid mediator that engages the cutaneous endocannabinoid system to modulate inflammation and barrier function
- Oligopeptide-1 (Epidermal Growth Factor, EGF) – supports keratinocyte proliferation and extracellular matrix synthesis
- Syn-Ake® (dipeptide diaminobutyroyl benzylamide diacetate) – a biomimetic peptide used to relax localized muscle contraction and improve vasodilation
- Helichrysum essential oil – provides antioxidant support and barrier protection
To date, no adverse effects have been reported across multiple PRiVIVE-based formulations, and in vitro testing on human skin tissue cultures has shown no irritation or structural tissue damage.
In practice, Juventix providers are using PRiVIVE as:
- A topical extension of Juventix Biotin PRP hair-restoration protocols (often combined with derma-rolling)
- A take-home maintenance serum to sustain growth-factor exposure between treatment sessions
- An adjunct following microneedling procedures of the scalp and skin
For hair restoration specifically, this creates a coherent continuum: in-office Juventix PRP + Biotin injections → immediate topical PRiVIVE application → ongoing home use of a PRP-customized serum, all while avoiding the mechanical trauma of needles at home and the risks of chronic acid-based exfoliation.
Adjunctive Technologies: LLLT and Exosome-Augmented Protocols
To further optimize outcomes, many practices layer Juventix PRP + Biotin and PRiVIVE with:
- Low-Level Laser Therapy (LLLT): Meta-analyses of randomized trials show LLLT significantly improves terminal hair counts in AGA and is well tolerated, particularly when used alongside other therapies.
- Exosome-enhanced strategies: Early clinical and preclinical work suggests exosomes derived from dermal papilla or mesenchymal stem cells can activate Wnt/β-catenin signaling, support anagen entry, and augment PRP responses in difficult hair-loss phenotypes. PRiVIVE is engineered specifically to accommodate PRP or exosomes in the same delivery platform, providing needle-free deployment of these autologous or allogenic biologics.
Key Clinical Takeaways for Providers Considering Alternatives to Glycolic Acid:
- Glycolic acid is not a hair-restoration therapy. Evidence for true regrowth is weak, whereas the potential for irritation, barrier damage, and worsening telogen effluvium is very real, particularly in already-sensitized scalps. (Source: NovaMane)
- PRP has evolved from “experimental” to evidence-based standard of care. Randomized trials and meta-analyses now consistently show improvements in hair density, with a safety profile that compares favorably to chronic pharmacotherapy.(Source: SpringerLink)
- Juventix PRP + Biotin is now backed by a U.S. Notice of Allowance for a non-provisional utility patent. The kit’s integrated biotin-within-the-tube design provides a differentiated, closed-system solution specifically engineered for hair restoration.
- PRiVIVE offers a needle-free, PRP- and exosome-compatible topical pathway. Dermal permeation data in human tissue models demonstrate platelet and protein delivery across intact skin without histologic damage, providing a rational alternative to acid-based “scalp peels.”
- A regenerative, multi-modal protocol aligns better with patient safety, pharmacovigilance trends, and long-term practice reputation than leaning on caustic chemical trends or single-agent pharmacologic solutions.
Ready to Compare Juventix in Your Own Hands? Here’s How:
- Request a FREE Biotin + PRP kit (just cover shipping & handling) ➜ https://juventix.com/sample-page/
Use the kit with your next appropriate hair-restoration candidate and consider pairing it with a custom PRP-enriched PRiVIVE™ Topical Serum as a take-home extension of care:
https://juventix.com/shop/privive-topical-serum-5-pack/
RESTORE, REVIVE, REGENERATE™ – JUVENTIX REGENERATIVE MEDICAL
References
- EMA PRAC Meeting Highlights – Suicidal Thoughts with Finasteride (2025). European Medicines Agency. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-5-8-may-2025 (European Medicines Agency (EMA))
- ClinCalc DrugStats—Finasteride Prescription Volume (2022). https://clincalc.com/drugstats/Drugs/Finasteride (Wiley Online Library)
- Gentile P., Garcovich S. Systematic Review of PRP in AGA (2020). Int J Mol Sci. https://www.mdpi.com/1422-0067/21/8/2702 (MDPI)
- Verma K. et al. PRP vs Minoxidil RCT (2019). J Drugs Dermatol. https://jddonline.com/articles/a-randomized-control-trial-comparing-the-efficacy-of-platelet-rich-plasma-and-5-topical-minoxidil-for-the-treatment-of-androgenetic-alopecia-S1545961623P0905X (ScienceDirect)
- Cruciani M. et al. PRP for Alopecia Meta-analysis (2023/2024). Aesthetic Plast Surg. https://pmc.ncbi.nlm.nih.gov/articles/PMC11073618/ (SpringerLink)
- Pillai J.K., Mysore V. Role of LLLT in AGA (2021). J Cosmet Dermatol. https://pubmed.ncbi.nlm.nih.gov/35283601/ (ScienceDirect)
- Zhang S. et al. Dermal-Papilla Exosome Study (2018). Stem Cell Res Ther. https://pubmed.ncbi.nlm.nih.gov/30924959/ (SpringerLink)
- Liu C. et al. MDSC-Exosome AA Model (2018). https://pmc.ncbi.nlm.nih.gov/articles/PMC8829028/ (SCIRP)
- Kwack M. et al. Exosomes Improve Hair Density (2024). https://link.springer.com/article/10.1007/s00266-024-04332-3 (SpringerLink)
- Almohanna H.M. et al. Biotin & Hair Loss Review (2019). Dermatol Ther. https://pubmed.ncbi.nlm.nih.gov/30547302/ (SpringerLink)
- PRP Therapy for Telogen Effluvium—Comprehensive Study (2024). J DERMIS (online). https://www.jdermis.com/full-text/platelet-rich-plasma-therapy-for-telogen-effluvium-a-comprehensive-evaluation-of-efficacy-and-safety (ScienceDirect)
- Tejapira K. et al. PRP in AA Systematic Review (2022). https://pubmed.ncbi.nlm.nih.gov/36507528/ (Wiley Online Library)
- Systematic Review PRP vs Minoxidil/Finasteride/Stem-Cells (2022). https://www.sciencedirect.com/science/article/pii/S235232042200102X (ScienceDirect)
- Lee Y. et al. Exosome-Augmented PRP Meta-analysis (2022). https://onlinelibrary.wiley.com/doi/10.1111/jocd.15869 (SpringerLink)
- PROSPERO-registered PRP Meta-analysis (2022). https://pubmed.ncbi.nlm.nih.gov/37533146/ (ScienceDirect)
Additional proprietary and technical information on Juventix PRP + Biotin Kit and PRiVIVE™ Topical Serum is drawn from U.S. patent documents and company clinical summaries.
Legal Disclaimer
The information contained in this article is provided for informational purposes only and should not be construed as medical or legal advice on any subject matter. You should not act or refrain from acting on the basis of any content included in this article without seeking medical, legal, or other professional advice. The contents of this article contain general information and may not reflect current legal developments or address your situation. We disclaim all liability for actions you take or fail to take based on any content of this article.